In the intricate world of cellular biology, one of the most fascinating processes that highlight how our cells recycle is autophagy. This fundamental mechanism involves the degradation and recycling of cellular components, ensuring that cells can maintain their functionality, respond to stress, and adapt to changing environments. As we delve into the remarkable journey of autophagy, we’ll explore its definition, historical significance, and implications for human health.
Understanding Cellular Recycling: The Mechanisms of Autophagy
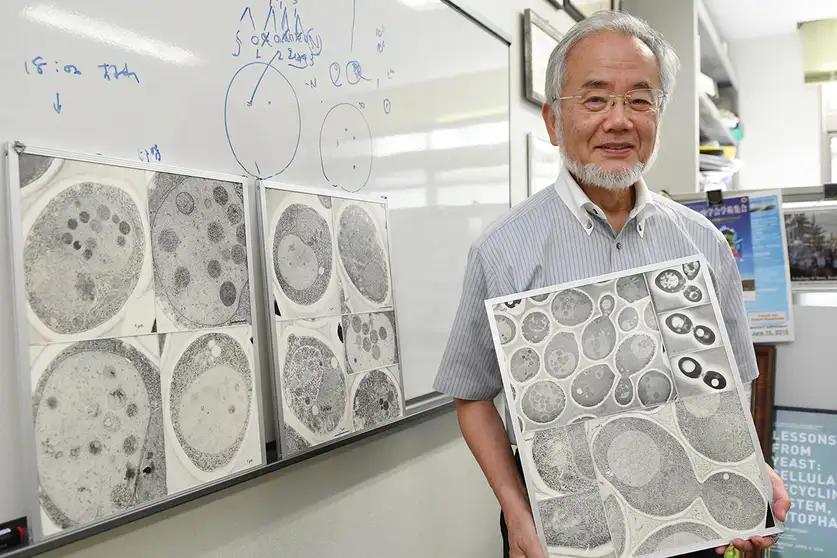
Autophagy, derived from the Greek words meaning “self-eating,” is an essential process in which cells digest their own components. This self-renewal mechanism plays a pivotal role in cellular maintenance, energy production, and response to various stressors. With the groundbreaking research conducted by Yoshinori Ohsumi, we have gained profound insights into this complex process and its life-sustaining functions.
Definition and Origins of Autophagy
At its core, autophagy refers to the cellular process by which cells break down and remove damaged or unnecessary components. This mechanism enables cells to recycle their internal contents, thus providing vital resources for new cellular structures and energy. Understanding how our cells recycle through autophagy sheds light on cellular resilience and adaptability.
The concept of autophagy was first proposed in the 1960s when scientists observed that cells could destroy their own components. Initially considered a survival strategy during starvation, autophagy has since emerged as a multifaceted process vital for cell health and function. The term itself reflects the intrinsic nature of this recycling process, where “auto” denotes self and “phagy” signifies eating.
Historical Context and Discovery
Yoshinori Ohsumi’s pioneering research in the 1990s marked a significant turning point in our understanding of autophagy. Utilizing baker’s yeast as a model organism, he identified key genes responsible for the autophagic process. Through his meticulous experiments, Ohsumi demonstrated that similar mechanisms operate in human cells, illuminating the universal importance of autophagy across species.
His groundbreaking findings laid the foundation for subsequent research exploring the role of autophagy in various physiological functions. Ohsumi’s contributions earned him the Nobel Prize in Physiology or Medicine in 2016, recognizing the profound impact of his work on our comprehension of cellular recycling.
Importance of Yoshinori Ohsumi’s Research
Ohsumi’s research transcends the academic realm; it has far-reaching implications for understanding human health and disease. By uncovering the molecular mechanisms underlying autophagy, he opened doors to potential therapeutic interventions for conditions linked to autophagy dysfunction. His work serves as a testament to the power of scientific inquiry and its potential to transform our approach to medicine.
As we unravel the complexities of autophagy, it becomes evident that this process is not merely a response to environmental challenges but also a fundamental aspect of cellular homeostasis. The ability of cells to efficiently recycle their components ensures that they remain agile and capable of responding to various stressors, thereby maintaining overall health.
Overview of the Cellular Recycling Process
The cellular recycling process initiated by autophagy is intricate yet elegantly organized. It begins with the formation of vesicles that encapsulate cellular debris, followed by transportation to specialized compartments within the cell for degradation. This process ultimately leads to the release of amino acids and other building blocks necessary for cellular renewal.
Understanding how our cells recycle through these mechanisms provides valuable insights into the interconnectedness of cellular function, metabolism, and overall health. The study of autophagy not only enhances our comprehension of basic biological processes but also paves the way for novel therapeutic strategies targeting various diseases tied to autophagy dysregulation.
The Process of Autophagy
Diving deeper into the mechanics of autophagy reveals a sophisticated array of steps and regulatory processes. The initiation of autophagy is carefully controlled, ensuring that cellular resources are utilized effectively.
How Cells Initiate Autophagy
The initiation of autophagy is triggered by several factors, including nutrient availability, stress conditions, and various signaling pathways. When cells experience nutrient deprivation or stress, they activate autophagy to conserve resources and eliminate damaged organelles.
During this initiation phase, specific proteins known as autophagy-related (ATG) proteins are recruited to form a phagophore, an early structure that surrounds the material destined for degradation. This carefully orchestrated event signifies the beginning of the recycling process.
The regulation of autophagy is nuanced, involving complex interactions between various signaling molecules. For example, the mTOR (mechanistic target of rapamycin) pathway acts as a critical regulator, inhibiting autophagy under nutrient-rich conditions while promoting it during times of scarcity. This fine-tuning ensures that cells adapt optimally to their environment.
The Role of Vesicles in Recycling
Vesicles play a crucial role in the autophagic process, serving as transport vehicles for cellular debris. Once formed, the phagophore expands and engulfs the targeted components, ultimately sealing to create an autophagosome.
These autophagosomes then fuse with lysosomes—organelles containing digestive enzymes—to form autolysosomes. Within this acidic environment, the contents are broken down, allowing for the recycling of valuable molecular constituents. This seamless integration of vesicular trafficking is pivotal for maintaining cellular integrity and function.
Degradation and Energy Production
The degradation of cellular components during autophagy serves multiple purposes. By breaking down damaged proteins and organelles, cells can eliminate potentially harmful materials and prevent cellular dysfunction. Furthermore, the recycling of cellular debris generates energy and metabolic substrates that fuel various cellular processes.
This energy production is particularly vital during periods of starvation or stress, enabling cells to sustain their functions even when external resources are limited. The ability to derive energy from internal sources highlights the adaptability of cells and underscores the importance of effective recycling mechanisms.
Building Blocks for Cell Renewal
Autophagy not only generates energy but also provides essential building blocks for cellular renewal. The breakdown of proteins and organelles yields amino acids, lipids, and nucleotides, which are crucial for synthesizing new cellular components.
This regenerative aspect of autophagy is significant for maintaining cellular homeostasis and promoting tissue repair. In times of stress or injury, the recycling of cellular components allows cells to rebuild and restore their functions efficiently.
Autophagy and Human Health
The implications of autophagy extend beyond basic cellular processes, significantly impacting human health. Dysregulation of autophagic mechanisms has been linked to a myriad of diseases, making the understanding of this process crucial for developing innovative therapeutic approaches.
Response to Starvation and Infection
One of the most well-studied aspects of autophagy is its role in responding to starvation and infection. During nutrient deprivation, autophagy is upregulated to provide energy and necessary substrates for cellular survival.
Moreover, autophagy plays a vital role in eliminating intracellular pathogens, such as bacteria and viruses. By encapsulating these harmful invaders in autophagosomes and delivering them to lysosomes for degradation, cells can effectively combat infections and maintain their integrity.
This dual role of autophagy as both a survival mechanism and a defense strategy highlights its importance in ensuring cellular health in challenging circumstances.
Autophagy in Development and Aging
Autophagy is also integral to developmental processes and aging. During embryonic development, autophagy contributes to the elimination of superfluous cells and the organization of cellular structures. This orchestrated removal of unwanted components is essential for proper tissue formation.
As organisms age, the efficiency of autophagy tends to decline, leading to the accumulation of damaged proteins and organelles. This age-related decline in autophagic activity has been implicated in various age-associated diseases, including neurodegenerative disorders and cancer. Understanding the relationship between autophagy and aging could pave the way for interventions aimed at promoting healthy aging and mitigating age-related diseases.
Genetic Diseases Linked to Autophagy Malfunctions
Mutations in genes associated with autophagy can lead to genetic diseases, underscoring the critical nature of this process for cellular health. Several hereditary conditions, such as certain forms of neurodegeneration, have been linked to disruptions in autophagic pathways.
For instance, impairments in autophagy can impede the clearance of toxic protein aggregates, contributing to the pathogenesis of disorders like Alzheimer’s disease and Huntington’s disease. By unraveling the connections between autophagy and genetic disorders, researchers aim to develop targeted therapies to restore autophagic function and alleviate disease symptoms.
Implications for Cancer, Parkinson’s Disease, and Diabetes
The relevance of autophagy extends into the realm of chronic diseases, including cancer, Parkinson’s disease, and diabetes. In cancer, autophagy can act as a double-edged sword—while it can suppress tumorigenesis by removing damaged organelles, some cancer cells may exploit autophagy to survive in hostile conditions.
Similarly, impairments in autophagy have been linked to neurodegenerative diseases like Parkinson’s disease, where the accumulation of misfolded proteins leads to neuronal death. Enhancing autophagic processes could hold promise for developing neuroprotective strategies.
In the context of diabetes, autophagy plays a role in maintaining insulin sensitivity and regulating glucose metabolism. Disruptions in autophagy have been associated with insulin resistance, highlighting the potential for targeting autophagic pathways in diabetes management.
Advances in Autophagy Research
Research into autophagy has rapidly advanced, providing new insights into its mechanisms and implications for health. Innovative techniques have emerged, allowing scientists to explore autophagic processes at unprecedented levels of detail.
Techniques for Studying Autophagy
The study of autophagy has evolved significantly, with the development of cutting-edge techniques that enable researchers to visualize and quantify autophagic processes in real-time. Methods such as fluorescence microscopy, electron microscopy, and live-cell imaging allow scientists to observe autophagic events directly within living cells.
Additionally, biochemical assays and molecular techniques facilitate the identification of autophagy-related proteins and genes, shedding light on the regulation of this process. These advancements have propelled our understanding of the intricacies of autophagy and its relevance to various biological contexts.
Discoveries Beyond Yeast: Human Applications
While much of the foundational research on autophagy was conducted using yeast, recent studies have expanded our knowledge to human applications. Investigators have increasingly focused on understanding how autophagic mechanisms translate across species, revealing shared pathways and regulatory networks.
Human cell models and animal studies have provided invaluable insights into the role of autophagy in various disease contexts. By elucidating the differences and similarities between yeast and human autophagy, researchers are better positioned to translate their findings into therapeutic strategies for human health.
Future Directions in Autophagy Research
The future of autophagy research holds immense potential, with many exciting avenues to explore. Scientists are investigating the interplay between autophagy and other cellular processes, such as apoptosis and inflammation, to gain a comprehensive understanding of cellular homeostasis.
Furthermore, the development of pharmacological agents that modulate autophagy presents a promising frontier in drug discovery. By harnessing the power of autophagy, researchers aim to develop targeted therapies for a range of diseases, from neurodegenerative disorders to cancer.
Conclusion
In conclusion, the mechanisms of autophagy illuminate how our cells recycle and maintain their health. Yoshinori Ohsumi’s groundbreaking discoveries have significantly enhanced our understanding of this essential process, revealing its implications for human health and disease.
As we continue to explore the complexities of autophagy, we unveil new possibilities for therapeutic interventions that harness the power of cellular recycling. Understanding how our cells recycle through autophagy not only enriches our grasp of cellular biology but also offers hope for addressing a myriad of health challenges in the future. The ongoing research in this field remains a beacon of innovation, paving the way for a healthier tomorrow.